OUR PROGRESS
We believe that people living with HIV have the potential to fight the virus, but they need a boost to win the war.
That’s why we’re pioneering a functional cure that amplifies the strength of the human immune system.

PHASE 1A DESIGN
What we set out to learn
First, we conducted a Phase 1a clinical study that evaluated the safety and durability of AGT103-T in seven people living with HIV. We also sought to evaluate the effectiveness of the modified T cells.
The participants received AGT103-T modified cells via infusion, followed by up to 180 days of monitoring post infusion for potential adverse events.
- Participant Screening
- Leukapheresis

Cell Manufacturing
& QC Testing

Infusion
180-Day post-
infusion follow up

01
- Participant Screening
- Leukapheresis
02
Cell Manufacturing
& QC Testing
03
Infusion
04
180-Day post-
infusion follow up
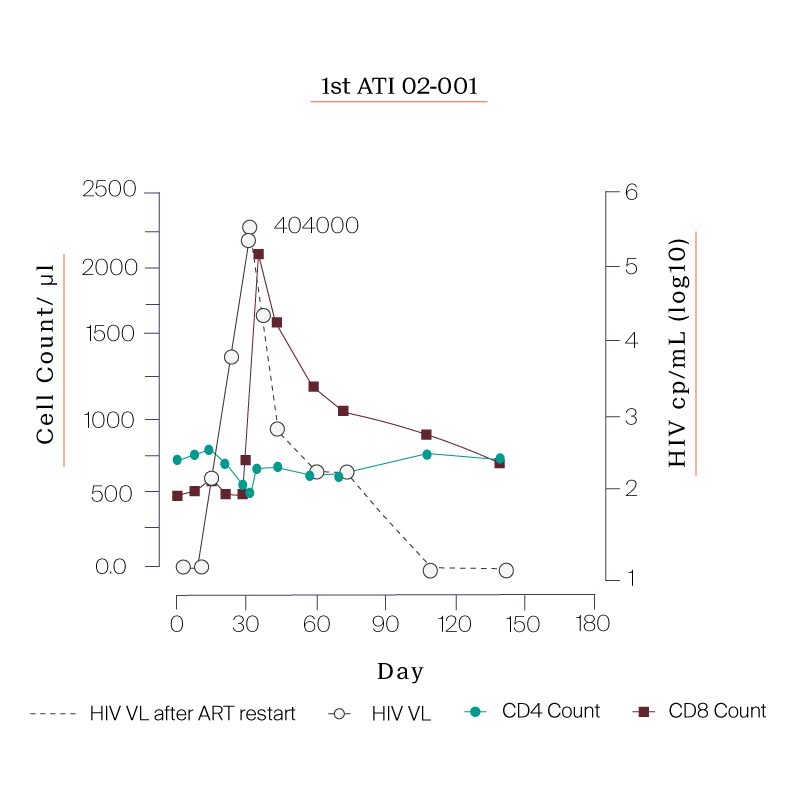
- a. The clinic failed to draw blood from 02-001 for research samples on the Day 180.
- b. Additional data unavailable at time of publication, see follow-on ATI study for additional detail.
- c. Day 90 sample for 02-009 arrived clotted and could not be processed.
PHASE 1A FINDINGS
What we observed
The Phase Ia data was encouraging across all three areas of evaluation:
- We observed no serious adverse events.
- The modified CD4 T cells were engrafted and persisted in all participants
- The CD4 T cells maintained their functionality
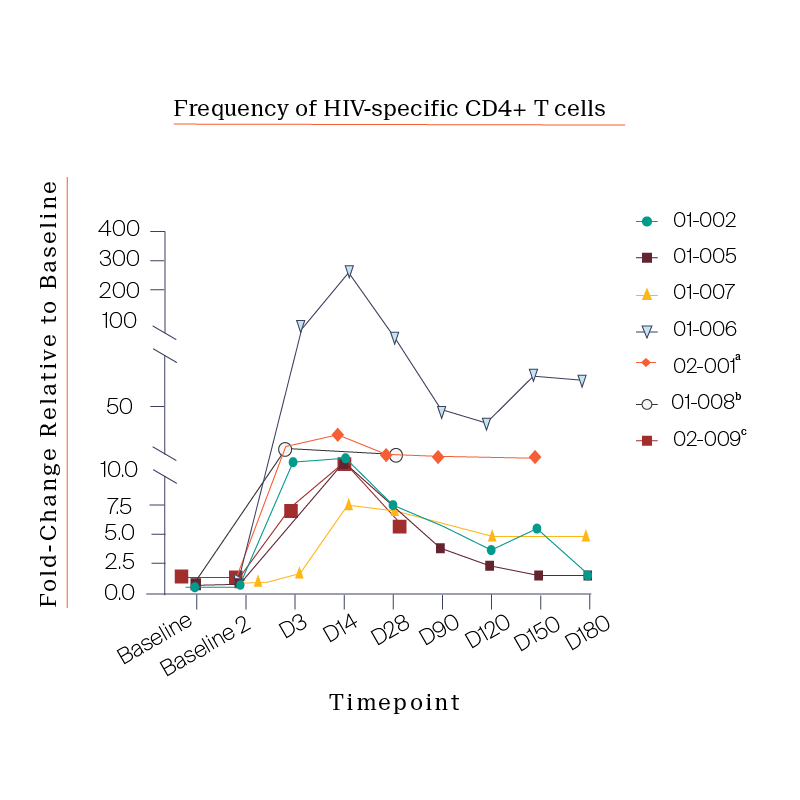
Notably, we also observed a consistent expansion of the CD4 T cells in patients that peaked approximately 14-days post infusion, an unexpected response that gave us key insight into how best to design the next trial protocol.
FOLLOW-ON ATI DESIGN
Building on our findings
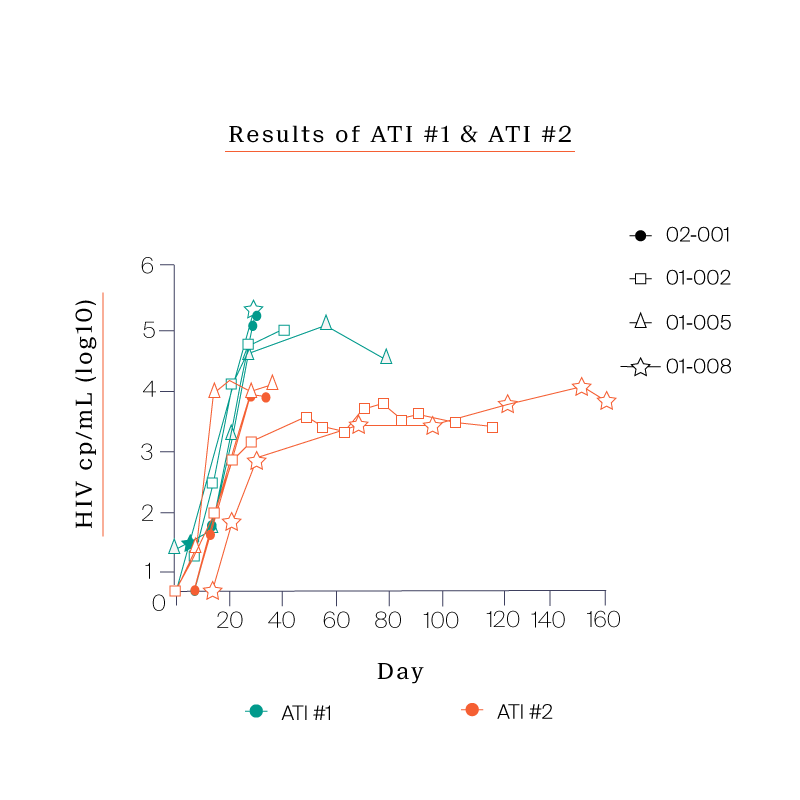
Following the Phase Ia study, we explored how the engrafted cells might impact their response to an analytic treatment interruption.
To do so, we designed a follow-on study with six participants from the Phase Ia trial. This new study featured an analytical treatment interruption (ATI) where we took the participants off of their antiretroviral therapy (ART) and evaluated their immune response to the virus.

Baseline criteria:
VL undetectable
CD4 counts normal
No infections

Cease NNRTI for seven days then all other drugs

Follow Viral Load and CD4 Count

Re-commence at any time or if VL > 100K X2 or CD4 < 350
01
Baseline criteria: VL undetectable CD4 counts normal No infections
02
Cease NNRTI for seven days then all other drugs
03
Follow Viral Load and CD4 Count
04
Re-commence at any time or if VL > 100K X2 or CD4 < 350
FOLLOW-ON ATI FINDINGS
What we observed
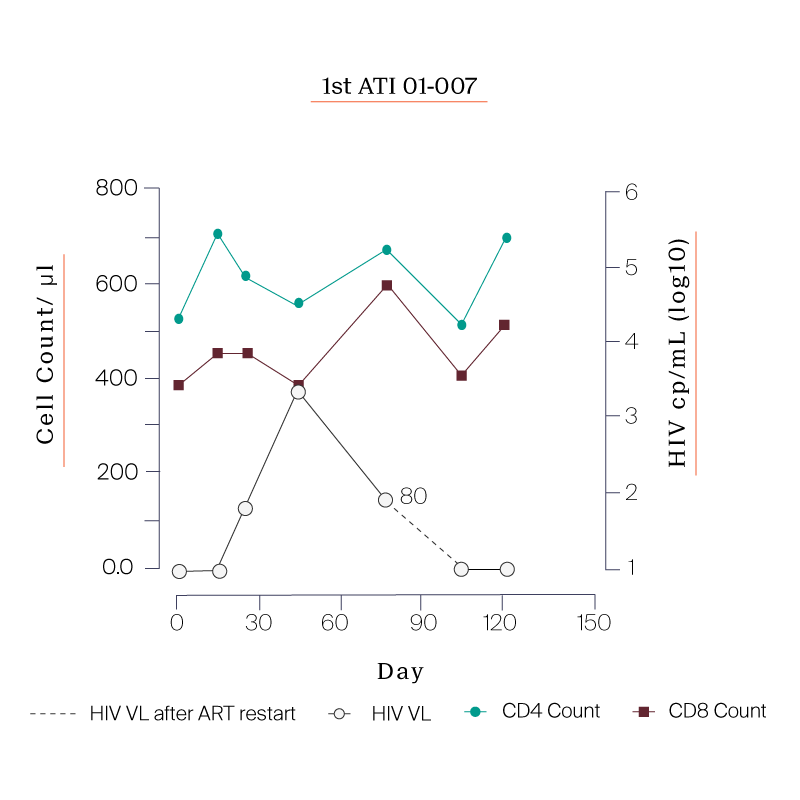
What we saw in all six study participants was quite unexpected. CD8 cell count increased after the viral rebound and CD4 cell count remained generally stable.
Following the first ATI, participants were put back on ART and were offered to go through a second ATI.
The reason for the second ATI was to see if the immune system would respond more effectively now that the cells had been exposed to the virus.
PARTICIPANTS
| Participant ID | Days Between Infusion and ATI Start |
|---|---|
| 01-008 | 150 |
| 02-009 | 99 |
| 01-002 | 490 |
| 02-001 | 246 |
| 01-005 | 411 |
| 01-007 | 390 |
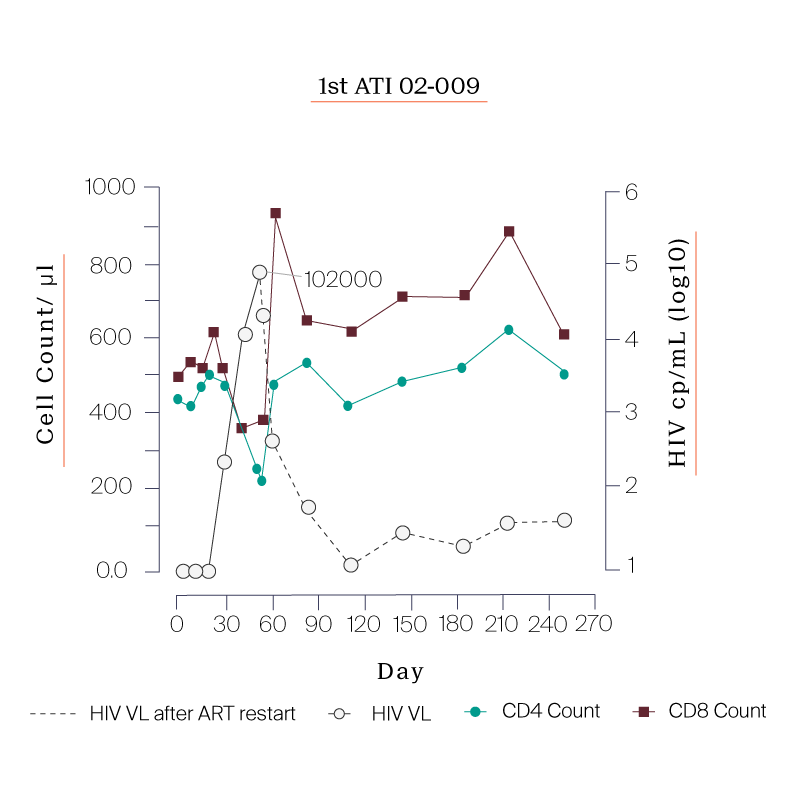
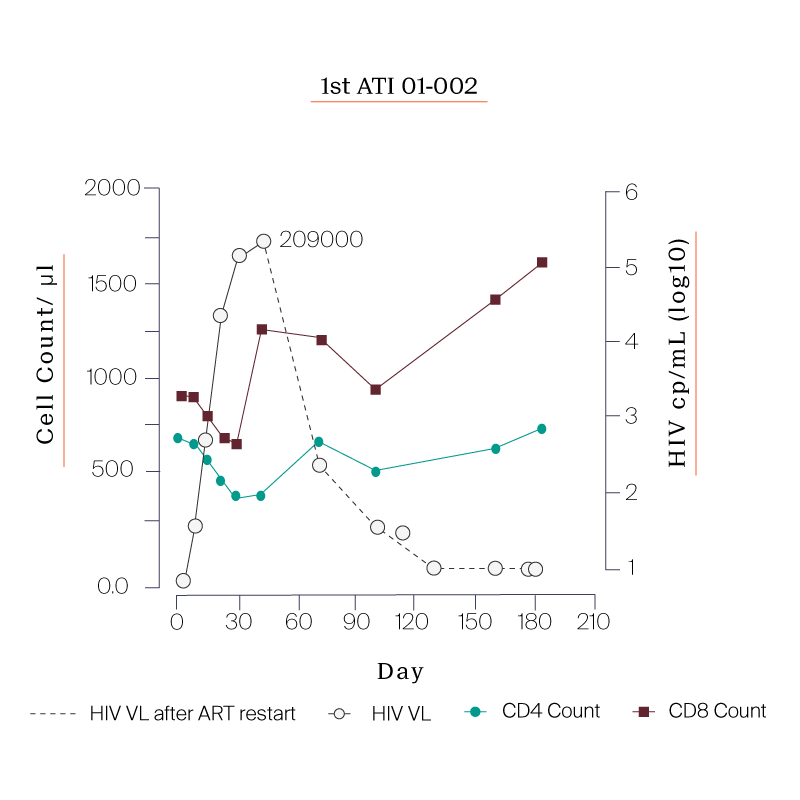
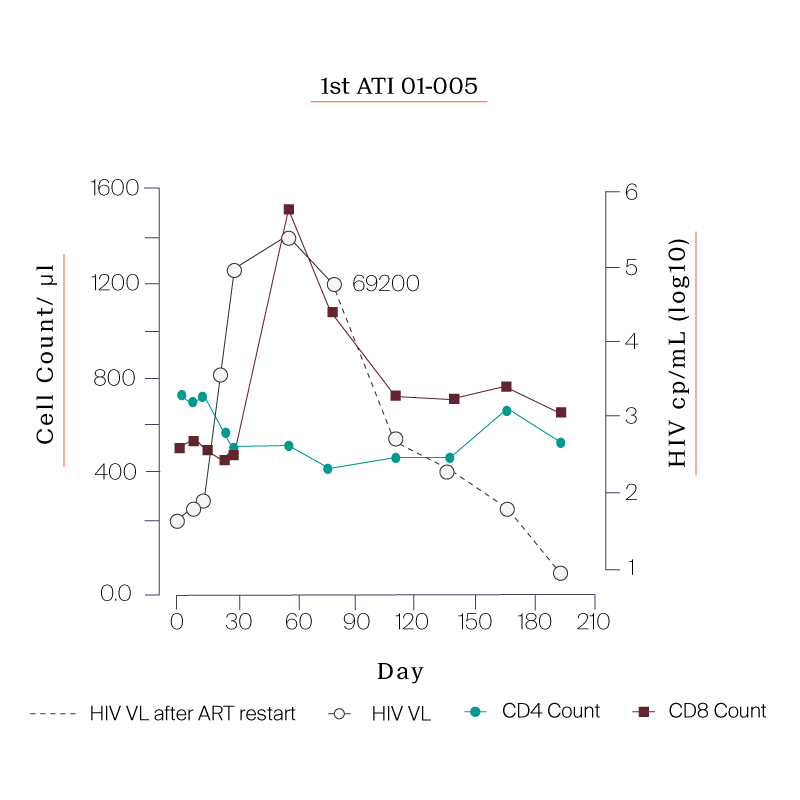
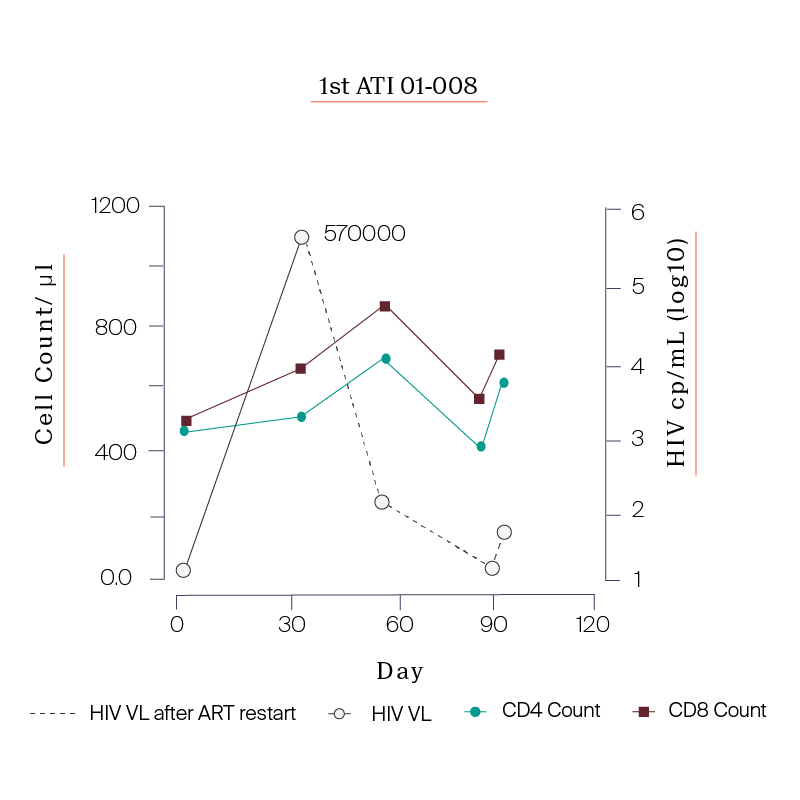
PARTICIPANTS
| Participant ID | Days Between Infusion and ATI Start |
|---|---|
| 01-008 | 150 |
| 02-009 | 99 |
| 01-002 | 490 |
| 02-001 | 246 |
IMPACT OF MULTIPLE ATIs
The potential of autovaccination
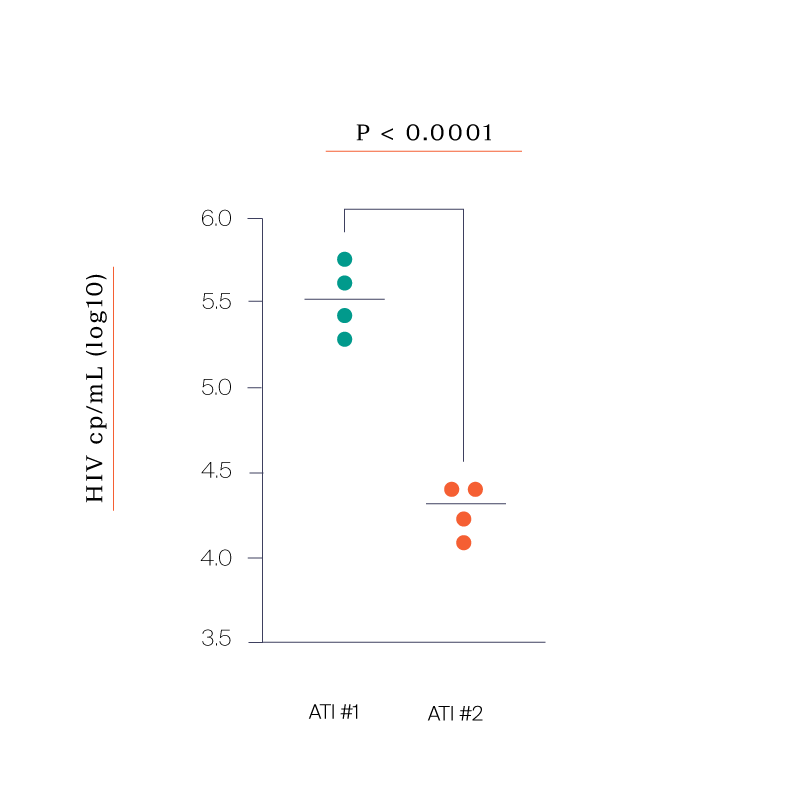
Four out of the six participants agreed to the second ATI. In all four participants we saw:
- Consistent CD8 and CD4 responses even more pronounced than the first ATI
- A significant reduction in peak viremia compared to the first ATI
- Set points that were all between 7,000 and 20,000 copies /mL