View the full Article on:
Addimmune
Author: Luke Williams

Why does HIV infect people for their entire lives?
We encounter countless unique viruses every day without falling ill, so what sets HIV apart? Why does this virus cause a lifelong infection marked by constant medication and social stigma? To form a solution, we need to start by understanding the problem. By studying how HIV establishes a persistent infection, and applying biological technologies to hinder that process, it may be possible to induce functional cure status in people living with HIV.
There are two key factors contributing to HIV’s ability to establish a lifelong infection: viral latency and viral reservoirs.
- Viral latency refers to the time lag between when a virus infects a cell and when it begins producing new viruses. Since HIV integrates its genetic material into the host cell’s genome, HIV-infected cells can persist in a latently infected state for a considerable period. These infected cells possess all the necessary instructions to make new HIV particles, hidden within their genes. When the cell begins reading those instructions, it begins producing new copies of HIV.
- A viral reservoir is the collection of cells that harbor latent infections in the body. In the case of HIV, the viral reservoir is primarily made up of Helper T cells, although other cell types like macrophages, CD127+ cells found in tissues such as lymph nodes, and other scattered cell populations may be involved.
To achieve a cure for HIV, scientists must address the viral reservoir. This involves identifying and characterizing all the cell types within the reservoir. Addimmune’s gene therapy for HIV, AGT103-T, addresses the reservoir by equipping immune cells with a defensive anti-HIV gene so they can move through the body identifying and responding to HIV-infected cells without succumbing to infection themselves. To help illustrate how it works, we’ll show you a new perspective on HIV, and elucidate how biotechnologies like ours can address chronic viral infections in new ways.
How do viral reservoirs work?
The broad definition of the term viral reservoir is an anatomical site where viruses accumulate and persist. Not all viruses cause persistent infections, but for the ones that do, the collection of cells which are permissive to long-term viral infection serve as a long-term source of the virus in the body. For example, the sensory neurons in the trigeminal ganglion are the primary site of Herpes Simplex Virus 1 (HSV1) latency. The collection of sensory neurons which are latently infected by HSV1 can read the viral genome upon the right stimulus and create new HSV-1 viruses, which then exit the sensory neuron and cause the characteristic cold sore.
Understanding the concept of the HSV1 reservoir is relatively straightforward since sensory neurons are stationary and the eruption of cold sores in the same anatomical area gives a spatial understanding of latently infected cells and viral reservoirs.
This example also highlights an important parallel to HIV: while antiviral medications can be taken to help alleviate symptoms during a cold sore outbreak, they cannot address the root cause of the problem: the latent HSV-1 DNA inside the sensory neurons. As long as the cells in the viral reservoir have the genetic instructions they need to assemble HSV-1, they may assemble new viruses when those instructions are read. Current medication can only hamper the assembly process, but cannot eliminate the genetic blueprint that resides within the latently infected cells.
To target viruses like HIV, HSV-1, and the many other viruses that establish persistent viral reservoirs, it is crucial to understand the details of viral latency and develop innovative approaches to target and eliminate the viral reservoir. By tackling the root cause of persistent viral infections, we can strive to develop more effective treatments and potentially find ways to eradicate these viruses from the body altogether.
What cells make up the HIV viral reservoir?
For HIV, the primary cell type that makes up the viral reservoir is the CD4+ “helper” T cell. These white blood cells play a crucial role coordinating the immune system’s response to pathogens. These cells are able to migrate throughout the body, rapidly divide in the presence of specific danger signals, and cluster closely to other helper T cells. The combination of these characteristics makes latently infected helper T cells especially difficult to handle, presenting a significant obstacle in the quest for an HIV cure.
In addition to CD4+ “helper” T cells, HIV can also establish latency in cells of the monocyte/macrophage lineage, as well as a few other scattered cell types. Understanding the diverse cell types involved in the viral reservoir is essential for developing effective strategies to combat HIV. By understanding the complexities of viral latency in these various cell populations, researchers can work towards developing innovative treatments that directly address the challenges posed by each cell type in the viral reservoir.
The tragic irony of HIV infection
Since HIV infects immune cells that are capable of rapid division, when latently infected cells copy themselves to form a pathogen-fighting army, they also unintentionally copy the HIV genome hidden in their genes. Through this natural immunologic process, the number of HIV-infected cells can increase without HIV needing to jump from one cell to another. These are the very immune cells which are responsible for antiviral responses, and on top of that, HIV preferentially infects the subset of helper T cells responsible for the anti-HIV response. This mechanistically reduces the number of immune cells participating in the anti-HIV response while bolstering the presence of the virus so it can infect other subsets of helper T cells.
Not only does this create the possibility of a surge in HIV-infected cells in response to infections, but it also creates a feedback loop. T cells respond to specific molecular patterns, and for the subset of latently infected T cells that respond to patterns associated with HIV, their antiviral response can inadvertently fuel a cycle of HIV reservoir expansion. This counterproductive loop hampers the natural immune system’s ability to fight HIV.
The ability for helper T cells to be promoted to long-lived “memory” T cells represents another challenge. Since memory cell populations can persist for a long time, they can harbor latent infections which factor into viral persistence. To help illustrate the concept, Figure 1, from Siliciano and Greene’s work, “HIV Latency” depicts the expansion of T cells in a normal scenario and an HIV-infected scenario:
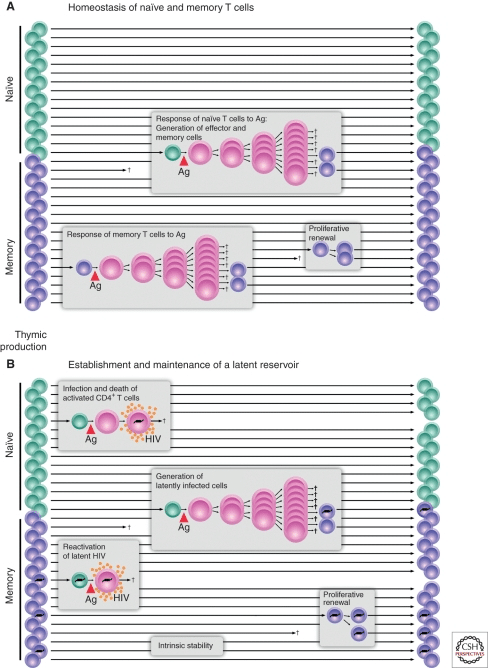
Figure 1: The top panel (A) shows the division and memory-formation activities of T cells in the body. The bottom panel (B) shows how HIV genes (the black lines inside of the T cells) can be amplified by this natural process. Photo credit: Siliciano and Greene
The top graph (A) shows how T cells behave in response to normal pathogens. Notice how naive T cells can divide and create large numbers of clones. After the primary immune response, some of those cells become memory T cells, denoted as purple cells. Those memory cells can then activate later upon stimulation, self-renew to maintain their population, or just remain in the body as a memory T cell for an extended period of time.
The bottom graph (B) shows the ways in which HIV can take advantage of this natural immunologic process. HIV can infect cells and kill them immediately, or it can remain wrapped in the genes of T cells as they transition into the memory T cell phenotype. HIV-infected memory T cells may persist in the body and may also divide to create more HIV-infected memory T cells. If HIV reactivates in these latently infected cells, they may die but may also produce a significant amount of HIV first.
The tragic irony in HIV infection is that T cells which are supposed to kill HIV become the kingpins of the infection. The natural ability for these T cells to persist for long periods of time and rapidly divide are hijacked by the virus. In the absence of a T cell response, the virus replicates unchecked, but when HIV-targeting T cells attempt to respond to HIV, they can become infected and then generate new HIV-infected T cells, further increasing HIV production in the body. This is checkmate for the natural immune system – so correcting the mechanisms driving this fundamental problem is a critical factor in any attempt to functionally cure HIV and enable clearance of the viral reservoir.
CCR5 Δ32
Thankfully, there are exceptions to the typical progression of HIV/AIDS. Some individuals carry a specific variant of the CCR5 gene known as CCR5 Δ32, which renders their helper T cells highly resistant to HIV infection. With these HIV-resistant T cells, their immune systems can remain uninfected while orchestrating a potent immune response against HIV. This effectively removes HIV’s advantage over the helper T cell and levels the playing field so the immune system can fight HIV like other viruses.
To complete the first half of its infectious cycle, HIV must:
- Enter a cell
- Convert its RNA genome into DNA
- Integrate that DNA into the cell’s genome
Then, there is the possibility of viral latency – the virus may reactivate after a variable amount of time post-infection. To continue the cycle, the next steps are:
- HIV genes are read to produce viral components
- These components are assembled to create new HIV particles containing RNA copies of the HIV genome
- These particles are exported from the cell to infect new cells
Cells with two copies of the CCR5Δ32 allele cannot produce a full-length CCR5 protein. Since HIV enters cells by attaching to CCR5 and CD4, the absence of functional CCR5 makes it significantly more difficult for HIV to enter these cells. The absence of full-length CCR5 on the cell surface presents a massive barrier to the viral cycle, allowing immune cells to coordinate the anti-HIV response without succumbing to infection and fueling the cycle of HIV progression.
Although other strains of HIV can use CXCR4 and CD4 to enter a cell, natural examples of HIV resistance show us the power of CCR5Δ32/Δ32 cells. In notable cases where HIV has been declared cured, such as the Berlin, London and Dusseldorf patients, CCR5Δ32/Δ32 cells played a critical role in their treatment plan. These real world examples show us the potent effects of protecting immune cells from infection so they can work unimpeded.
How to address HIV’s viral reservoir
So, how do we confront a virus that silently hides within the genes of cells which are scattered throughout the body, ready to reactivate at any time? In the few cases where HIV has been declared cured, doctors used radiation and chemotherapy to eliminate a substantial portion of the viral reservoir. They then introduced a new HIV-resistant immune system via CCR5Δ32/Δ32 stem cell transplant. For more information on those cases, read Challenge the Narrative: HIV is a Curable Condition.
Case studies such as the Boston Patients and the Minnesota Patient show us that simply depleting the viral reservoir with radiation and chemotherapy is insufficient, as HIV rebounds if it encounters susceptible helper T cells. The introduction of CCR5Δ32/Δ32 cells is a critical factor that differentiates viral rebound from a potent antiviral immune response with the potential to functionally cure HIV infection.
HIV-resistant T cells have a profound impact on HIV infections. They can navigate throughout the body, rise in number in response to HIV presence, and orchestrate a precisely targeted immune response against HIV-infected cells. In cases like the Berlin, London, New York, City of Hope, and Dusseldorf patients, HIV-resistant helper T cells induced durable HIV remission. Some patients have been declared cured after years of remission, while others are still being monitored to assess their eligibility for a cure declaration.
The methods used to induce HIV remission in these patients are not scalable for various reasons, but the message is clear: HIV can be cured. Building on these successful cases, Addimmune is progressing through human trials for AGT103-T, a gene therapy for HIV. This therapy aims to replicate the effects observed in the aforementioned patients in a safer and more scalable manner. By simply collecting a blood sample, we can isolate regular helper T cells and stimulate the subset of T cells that respond to HIV. Next, our gene therapy transforms those HIV-responsive T cells into AGT103-T cells, which are equipped with specialized anti-HIV defenses. Infusing those AGT103-T cells back into the patient may offer curative benefits without the need to deplete their immune systems or rely on the supply of rare, naturally-sourced CCR5Δ32/Δ32 cells.
Addimmune is currently conducting human trials for AGT103-T and hopes to demonstrate the safety and scalability of this approach to HIV infection. The viral reservoir has long been a challenging hurdle in the pursuit of a cure, and we sincerely hope that AGT103-T cells will prove their ability to recognize and respond to the viral reservoir in ways that traditional medications simply cannot. If you are interested in following our progress, supporting our cause, or learning more about gene and cell therapies, we encourage you to sign up for our newsletter, follow us on social media, and help spread the word! A technological revolution is on the horizon in the field of medicine, and we invite you to join us on this transformative journey.
Additional Questions
Thanks for reading to the end! Here are some common follow-up questions:
Since the HIV viral reservoir is so elusive, does that mean it is impossible to cure HIV?
Answer: We believe HIV is a curable condition, even though the viral reservoir is so difficult to identify and address. Natural examples of viral suppression of HIV show that the immune system is capable of exerting sustained pressure on the cells of the viral reservoir – as seen in elite controllers and long-term non-progressors. Additionally, a growing number of case studies have successfully proven the concept that HIV can be cured, though the methods used in those cases are not commercially viable due to scalability, ethical concerns, and risk to the patient.
Nevertheless, these proofs of concept provide a basis for HIV cure attempts like ours, showing us that success is possible, and what that looks like so we can fine tune our methods with the goal of inducing functional cure status in as many patients as possible.
How long can HIV be dormant?
Answer: This question has multiple answers. On the basis of an individual cell, the HIV provirus can remain inside an uninfected cell for a variable amount of time, depending on environmental conditions and where the HIV provirus integrates into the cell’s genome. For an interesting perspective on this, consider reading the work of Jassens et al, who manipulate the integration sites of HIV-1 to study how retargeting HIV-1 integration events away from their naturally preferred sites affects the length of latency.
On the basis of an entire population of HIV-infected cells, HIV proviruses can persist for an entire lifetime. Since HIV-infected cells copy their entire genome (including the integrated HIV provirus) when they divide, HIV proviruses can be generated without new infections occurring. Additionally, since HIV can be latent in memory T cells, the reservoir of HIV proviruses can last a long time for the same reason that immune memory lasts a long time.
Why is HIV so hard to treat?
Answer: HIV infection is often asymptomatic, so it is difficult to identify before the virus entangles itself with the immune system. Additionally, HIV mutates very quickly, so doctors need to consider the possibility of antiviral resistance. Beyond the scientific perspective, doctors and public health officials also need to consider the economic and social factors of HIV infection that contribute to the epidemic.
HIV is difficult to treat because no matter what perspective you use to analyze HIV, it’s a tricky virus. Medication can be expensive, people who are infected may not have access to testing, or may be in denial due to the stigma associated with infection, the virus creates a long-lived reservoir, and that reservoir can be established during a primarily asymptomatic phase.
