View the full Article on:
Addimmune
Author: Addimmune Staff

What is gene therapy and why is it useful for HIV infection?
Gene therapy is a technique used by scientists and doctors to deliver medicinal genes to a patient’s cells. With today’s technological advancements, it is possible to identify the malfunctioning gene responsible for a given disease and then correct it using advanced genetic tools. With gene therapy, scientists can fix the root cause of the malfunction and create cells that manage a patient’s disease instead of relying on constant medication. This modern treatment modality allows patients to live normal lives without the side effects of traditional medicine, and brings previously incurable conditions into striking distance.
The obvious use case for gene therapy is in addressing inherited genetic disorders, but since HIV integrates its DNA with our own DNA when it infects susceptible cells, it can also be argued that HIV causes a non-inherited genetic disorder. Recognizing the opportunity to combat HIV at the genetic level, our team began designing AGT103-T. With careful analysis and creative application, we have developed a gene and cell therapy that we believe can correct HIV infection. Here, we’ll show you how we do it.
What AGT103-T does to fight HIV
For the vast majority of people, exposure to an infectious dose of HIV leads to infection, which if left untreated, inevitably leads to AIDS. HIV infects and kills the very cells that are responsible for generating a long-term immune response to fight the HIV infection. This means that as the virus gains prevalence, the body loses its ability to fight it; an unbreakable cycle in most cases. However, there are people whose immune cells are resistant to the HIV infection, exposing key weaknesses of HIV and providing researchers with actionable examples on how to defeat the virus.
AGT103-T is a gene and cell therapy made up of helper T cells which have been given specialized anti-HIV genes. Helper T cells are central to the adaptive immune system, and the primary target of HIV infection, so protecting these cells allows them to coordinate an anti-HIV response without simply becoming infected. Since T cells can divide, persist and can circulate throughout the body, the AGT103-T cells are able to form a self-sizing army of HIV-resistant T cells which can recognize and respond to HIV wherever it is in the body.
CCR5 Knockdown
CCR5 is a human protein which spans the cell membrane, allowing cells to detect messenger molecules like CCL3, CCL4, and CCL5 outside of the cell, then cause a change within the cell. While CCR5 is an important protein for immune cells like helper T cells, HIV can use CCR5 as a co-receptor to enter cells. Recognizing that CCR5 is an important part of a cell’s susceptibility to HIV infection, doctors and scientists tested the hypothesis that immune cells without canonical CCR5 could resist HIV infection.
Indeed, in late 2010, Timothy Ray Brown, previously known only as the “Berlin Patient”, made headlines as the first person to be cured of HIV. As a treatment for his leukemia, Brown received a bone marrow transplant from a donor with a natural mutation in CCR5, which in turn, resulted in an immune system with cells that did not produce full-length CCR5. Among these cells were HIV-resistant helper T cells which could coordinate an antiviral immune response and successfully fight the remaining HIV in Brown. Today, the Berlin Patient is joined by the London, New York, City of Hope, and Dusseldorf patients – who all had variations of the CCR5-null stem cell transplant procedure.
Clearly, a population of helper T cells without canonical CCR5 is critical to any attempt to cure HIV with the immune system. For this reason, we engineered AGT103-T cells with a microRNA which knocks down CCR5, rendering the T cell highly resistant to HIV infection. MicroRNAs are precision tools that decrease the expression level of certain genes by “intercepting” mRNA before it can be translated into protein. Since microRNAs bind to RNAs with complementary sequences, a well-designed microRNA does not bind to the mRNAs of other cellular genes, but specifically leads to the degradation of its target mRNA. In this case, the microRNA blocks mRNA that would have otherwise told the cell to make CCR5.
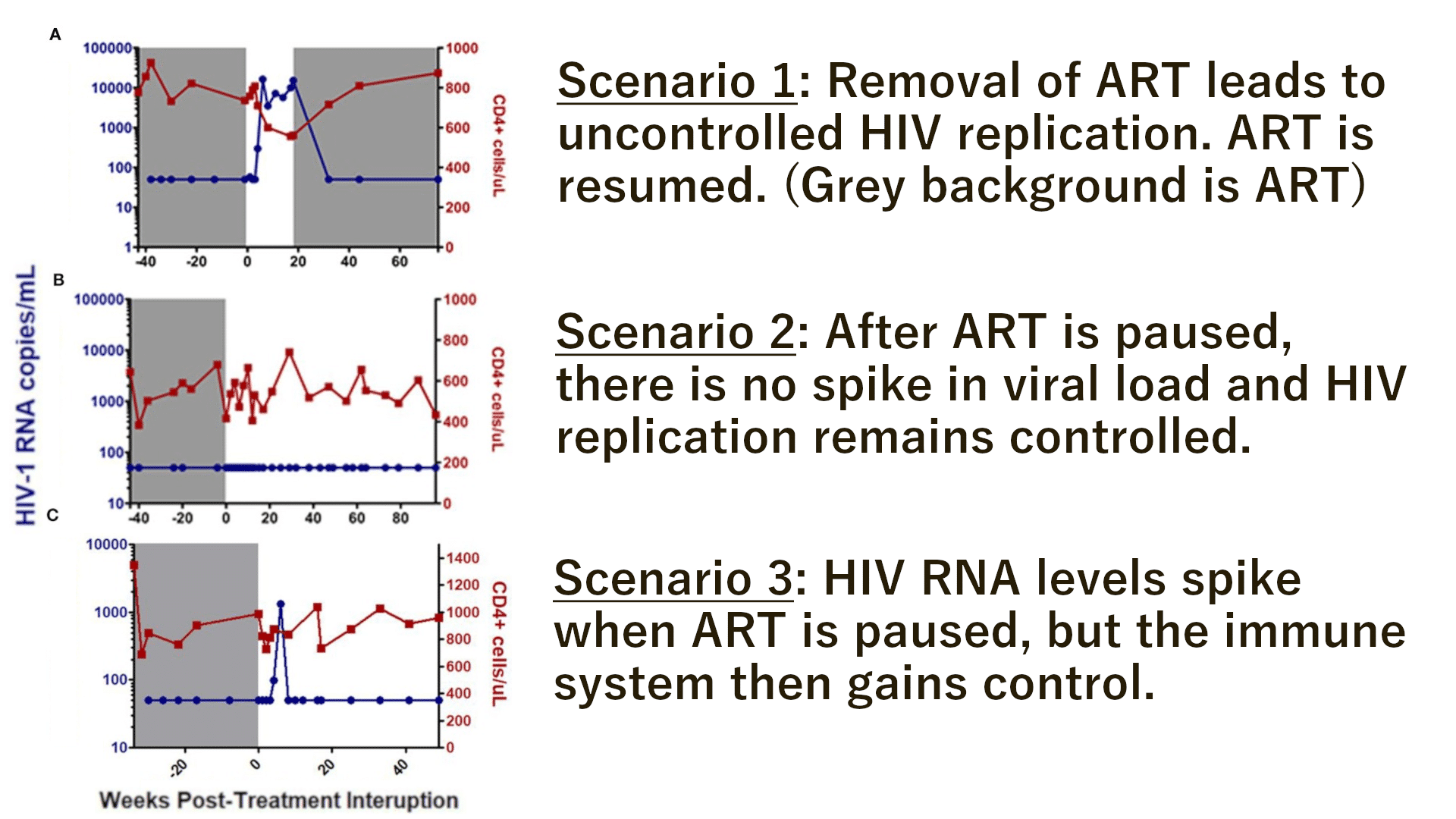
Figure 1: This diagram from the NIH’s National Library of Medicine depicts RNA interference. Short interrupting RNAs (siRNAs) and microRNAs (miRNAs) are important mechanisms used by human cells to regulate what proteins the cell can make, and in what amounts. By taking advantage of this pre-existing mechanism and simply directing it against CCR5, we can drastically reduce the amount of CCR5 that is produced by the cell, decreasing its susceptibility to HIV infection.
