
View the full Article on:
Addimmune
Author: Addimmune Staff

If HIV is incurable, why have some people been cured?
Under normal circumstances, exposure to HIV leads to infection, which inevitably leads to AIDS after years of virus-mediated destruction of the immune system. However, there are some exceptions to this rule, and by studying these exceptions, researchers have successfully cured HIV infection on multiple occasions. These cases challenge the commonly accepted idea that HIV is an incurable condition, and by understanding more about these rare cases, we can construct the argument: “HIV is curable, under specific conditions.” Understanding and replicating those conditions is therefore central to the development of a cure for HIV.
The Berlin Patient – Timothy Ray Brown
It had been known for some time that certain people were able to resist infection by HIV. But their resistance was attributable to their genetic makeup or their immunologic function, so HIV resistance was thought to be inherent to the individual. However, in the case of the Berlin Patient, it became apparent that HIV resistance could be transferred via transplantation of HIV resistant cells from a donor.
Despite its extreme versatility, HIV can only infect a handful of cell types, principally helper T cells, some other immune cells, and a few other scattered exceptions. Since infecting the helper T cell had the most pronounced effect on the immune system, it was theorized that if a patient’s HIV-infected helper T cells were replacing with new, genetically resistant helper T cells from an HIV-resistant donor, it may be possible to cure infection – but that was much easier said than done. The main challenges were:
- Since HIV can entangle itself into the genomic DNA of cells and lay “dormant” for an undetermined amount of time, you would realistically need to wipe out all helper T cells indiscriminately in order to get all the HIV-infected cells. This would cripple the immune system.
- Replacing a person’s immune system with donor cells means that the donor needs to be a very close genetic match in order to avoid problems.
- You can’t just uproot an immune system and give it to another person, instead, you’d need to transplant the “seed” and let a new immune system grow from it. Bone marrow contains the stem cells to the immune system, so a bone marrow transplant, or other type of stem cell transplant would be required. These procedures can be invasive and are not performed unless they are necessary.
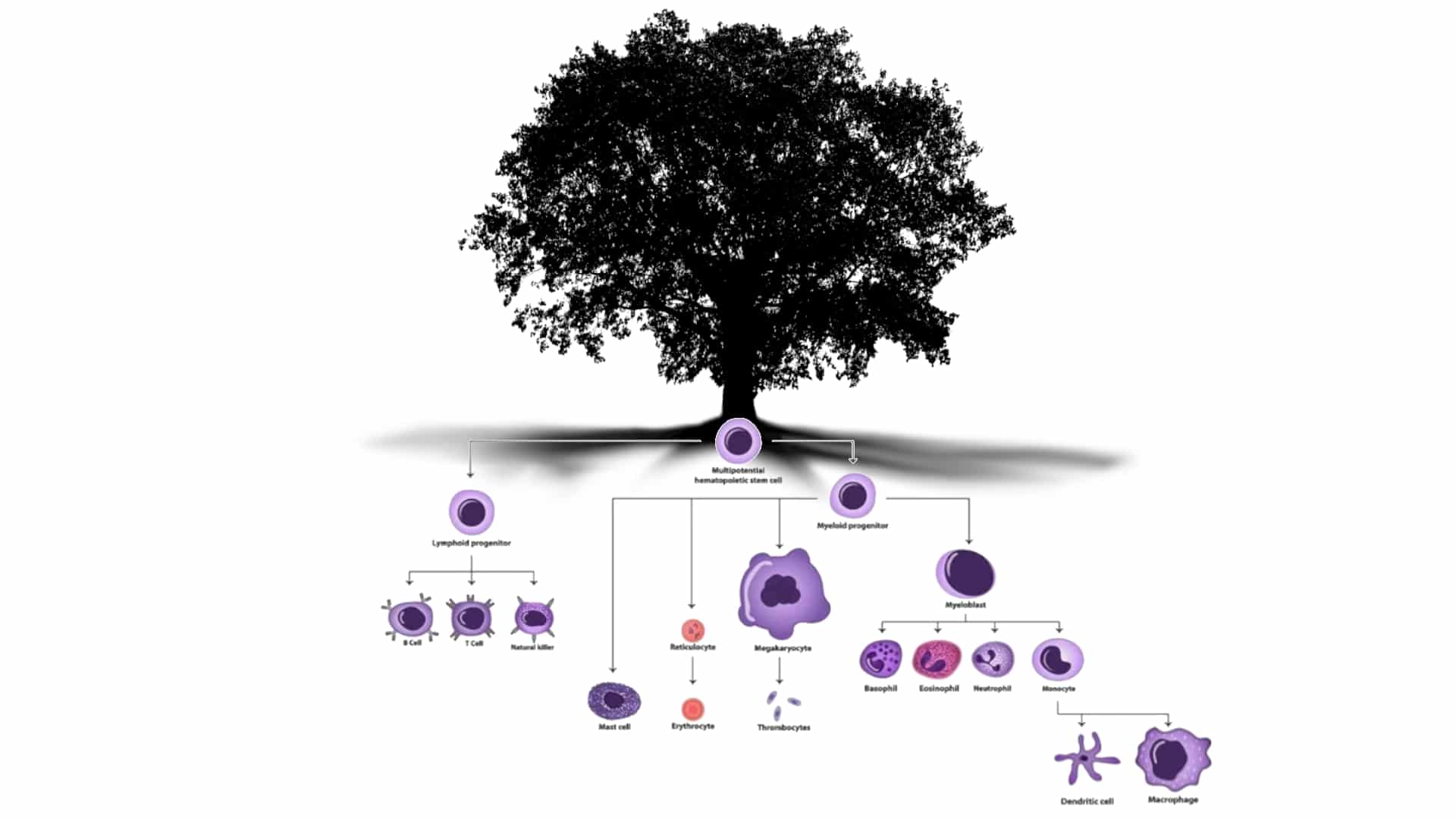
Figure 1: The immune system flourishes from the various cell types that derive from HSCs. If new HSCs are installed in a patient, the new immune system will have the properties of those HSCs. Photo adapted from the work of Maryam Mahdi.
The Berlin Patient, who later revealed his identity as Timothy Ray Brown, was a person living with HIV who developed acute myeloid leukemia, a form of blood cancer. Doctors needed to kill Brown’s cancerous white blood cells before those cells could kill Brown. Since Brown had HIV-infected white blood cells as well as cancerous white blood cells, the radiation and chemotherapy which killed his existing white blood cells fulfilled a dual purpose. After wiping out those cells, Brown had a much lower cancer burden as well as a lower HIV burden, but he did not have an intact immune system – a problem which required solving.
Luckily for Brown, Dr. Gero Huetter had a plan to solve two problems at once. Luck and creativity coincided when Dr. Huetter was able to procure bone marrow from a donor who was a close match to Brown with one key difference – the donor was genetically resistant to HIV. With this bone marrow, a new HIV-resistant immune system would arise within Brown to replace the cancerous one that had been killed with radiation and chemotherapy. In theory, the new system of HIV-resistant cells would be able to fight any remaining traces of HIV. Unfortunately for Brown, the first transplant did not take hold, necessitating another transplant, but through a strong will and good care, Brown fought through the aftermath of these procedures.
After a long recovery period, an abundance of caution and batteries of tests, Brown was declared cured of HIV. The declaration was groundbreaking: for the first time, HIV infection was not an absolute. It was indeed curable, though the methods were admittedly unscalable. Nonetheless, replicating the conditions seen in Brown’s case seemed like a promising route toward a cure, and scientists and doctors soon began repeating these methods.
The London Patient – Adam Castillejo
An explosion of HIV cure research followed the case of the Berlin Patient, and after 10 years of effort, the London Patient was identified. Adam Castillejo, who had undergone treatment for Hodgkin’s lymphoma, was experiencing remission from HIV after a bone marrow transplant containing HIV-resistant stem cells. Just like with the Berlin Patient, these HIV-resistant stem cells were giving rise to an HIV-resistant immune system which was able to clear the HIV reservoir. After intense study, the London Patient became the second patient to be declared cured of HIV, perpetuating a trend which would only increase in pace with further research.
The London and Berlin patients underwent slightly different procedures, but the uniting factor between them was the introduction of stem cells with the CCR5Δ32/Δ32 genotype. Since HIV uses CCR5 as a coreceptor to enter cells, immune cells with a truncated CCR5 (this is what the CCR5Δ32/Δ32 genotype results in) tip the playing field in the immune system’s favor. These cells can coordinate an immune attack on HIV but resist infection by HIV, removing the advantage that HIV has over an average immune system.
The effectiveness of CCR5Δ32/Δ32 cells is best illustrated by the “Minnesota Patient” and two separate “Boston Patients” who were treated for blood cancer, but received stem cells without the CCR5Δ32/Δ32 genotype. In these patients, HIV relapsed after an average time of 199 days.
The Berlin Patient showed that curing HIV was possible, and the London Patient showed that the methods were replicable, but the scalability of these methods was still a problem. Individuals with two copies of the delta32 mutation, denoted as CCR5Δ32/Δ32, are rare to begin with, and in order to safely transplant their bone marrow into another person, that person must be a close genetic match to avoid rejection, among other post-transplant complications. Additionally, CCR5Δ32/Δ32 stem cells did not have a perfect record, as seen in the Essen Patient, whose HIV was able to work around these cells and relapse, showing that these cells are more of a tool with strong potential if used in the right context, and less of a cure-all. In combination, these factors barred CCR5Δ32/Δ32 stem cell transplantation from becoming a viable option for the millions of people living with HIV.
The New York Patient
Since the rare CCR5 Δ32 allele is mainly found in northern european populations, and matching donors to recipients gets more difficult as their genetic backgrounds became more distant, researchers were interested in using new transplantation techniques to overcome these barriers. Dual stem cell transplant, and particularly haplo-cord transplant techniques have recently been studied at the Weill Cornell Medical College, where the New York patient was treated. By transplanting two sources of blood stem cells, which in this case were:
- Adult hematopoietic stem cells from a related or otherwise closely matched donor
- Imperfectly matched CCR5Δ32/Δ32 stem cells from umbilical cord blood (UBC)
Researchers were able to enable a faster blood cell count recovery with lower rates of graft-versus host disease and malignancy recurrence. Since the UBC cells engrafted well into the New York patient (100% chimerism by week 14) once the immune system recovered, it was populated by CCR5Δ32/Δ32 cells which are resistant to HIV infection. According to the World Health Organization (WHO),
“This dual transplant process has been used in some individuals with high-risk cancers and requires less restrictive human leucocyte antigen (HLA) sample matching than adult- stem cells-only transplants, and it also makes the transplant procedure faster and safer.”
The dual stem cell transplant technique was developed primarily for cancer treatment, but has important implications for HIV remission. Since the CCR5Δ32/Δ32 genotype is very rare, even if a donor is found, their cells may not be matched closely enough to the recipient for a normal transplant. Using this technique, doctors could open the CCR5Δ32/Δ32 stem cell transplant approach to a wider range of people living with HIV who also need a stem cell transplant for cancer. The New York Patient, who is being studied as another HIV cure case, was especially difficult to match for donor cells, but was able to receive a transplant using this technique. The WHO continues:
“In this case, the cells of the umbilical cord had the CCR5 mutation and the adult stem cells also didn’t require an identical HLA matching, which is especially difficult to get for patients of African or mixed-race ancestry.“
The researchers freely admit that the approach is invasive, complex and risky – and that even with the increased availability of half-matched stem cells, transplantation techniques still would not scale to the millions of people living with HIV since the CCR5Δ32/Δ32 genotype is only present in ~1% of the population. Nonetheless, The New York Patient is further proof that HIV can be cured, and that variations on the original methods used on Timothy Ray Brown can augment the availability of this procedure.
The City of Hope Patient
In 2022, AJMC reported on the fourth case of HIV remission after a bone marrow transplant, The City of Hope Patient. This case provided yet another instance proving that it is possible to cure HIV, but what really made the case special was the patient’s age. At 63 years old, a man living with HIV received a bone marrow transplant for acute myelogenous leukemia, and by age 66, his HIV was still in remission after having stopped ART in 2021.
Place yourself in the shoes of The City of Hope Patient – imagine you are a man who lived through the early days of the HIV epidemic, watched friends and acquaintances fall ill and possibly lose their lives, witnessed battles with the FDA over fast tracking medication, observed how pharmaceuticals evolved to become more effective with fewer side effects. Then, after bearing witness to the arc of HIV history and facing incredible social stigma, you find yourself in remission without the need for antiretrovirals. According to the AJMC, the patient said in a statement:
“I never thought I would live to see the day that I no longer have HIV.”
As the fourth person to achieve long-term HIV remission from stem cell transplantation, the City of Hope Patient adds to the mounting evidence which identifies HIV as a curable condition. While the methods are dangerous and unscalable, the fact remains that if any company were able to develop safer, scalable methods to induce similar effects in people living with HIV, there is a solid precedent for an HIV cure, and so many people whose lives would be forever changed.
The Dusseldorf Patient
Early in 2023, a fifth person was declared cured of HIV. The Dusseldorf Patient is a man named Marc who was a part of the amfAR ICISTEM cohort that includes the London Patient. Marc was living with HIV and taking ART when he received a CCR5Δ32/Δ32 bone marrow transplant for acute myeloid leukemia in 2013. Unfortunately, his leukemia returned, but doctors were able to
successfully treat it without any additional stem cell transplantation. In 2018, Marc was ready to discontinue his ART and, after 5 years of study, he was declared cured of HIV in 2023.
When can everyone else be cured of HIV?
From each of these cases where patients exhibit years of HIV remission without ART, or are simply declared cured, the medical community is left in a conundrum: how do we process and move forward with this data? Left untreated, HIV is both infectious and lethal, placing a heavy burden of proof on studies which aim to liberate patients from their HIV infections. However, there is undeniable evidence that HIV is indeed a curable condition, which could lead to a future where patients can undergo treatment to permanently cure HIV infection. In order to make that future a reality there are two main obstacles which will need to be addressed:
- Create a safe, scalable method to recreate the conditions seen in the patients who have been cured of their HIV infection. Addimmune, a gene and cell therapy company, is currently in human trials for AGT103-T, a gene therapy for HIV which was designed as a functional cure for HIV infection. Addimmune’s sole mission is to fulfill this unmet medical need.
- Through consistent data, ease the concerns of the medical community. Whether it’s social stigma or professional caution from healthcare providers, the perception of HIV will need to change in step with medical interventions. Thankfully, the cure for Hepatitis C created a precedent for the transition from a stigmatized, lifelong infection to a curable condition.
So long as these two conditions are fulfilled, we can change the lives of millions of people who are living with HIV. Through Addimmune’s clinical trials for AGT103-T, we hope to satisfy the first condition, and should we be successful in our venture, the next step is simply to spark a conversation. To fulfill the second condition, we need the help of the community. Whether you’re a patient, doctor, advocate, investor, or simply someone who is interested in medical progress, we need your voice. Together, we can write a future with fewer incurable diseases, and less stigma towards the people who are affected. Join us in observing the clinical progress of AGT103-T, and use your voice to change hearts and minds so we can finally liberate the people who have suffered silently through an epidemic spanning decades.
Further questions
Thanks for reading till the end! We understand that HIV science can be tricky, so please take a look at some common follow-up questions and answers.
Question: If the bone marrow transplant patients were treated with chemotherapy and radiation to kill their white blood cells, wouldn’t that kill all their HIV-infected cells?
Answer: One of the reasons HIV is so difficult to cure is viral latency, the lag between when a cell gets infected and when it begins producing HIV, which can be anywhere from hours to months. These latently infected cells can be any cell type that is susceptible to HIV, so while many of them would be the helper T cells (which HIV preferentially infects) other cell types may also be present in the collection of latently infected cells: the viral reservoir.
When doctors treat certain forms of blood cancer with radiation and chemotherapy, they are trying to wipe out a certain cell type since some of those cells have gone cancerous. While a large portion of the viral reservoir may be cleared by radiation and chemotherapy, other cell types with latent HIV infections may remain and reactivate later. This is likely the reason why the Minnesota Patient and the two Boston Patients had a viral rebound whereas the London and Berlin Patients did not. The difference between those patients is the CCR5Δ32/Δ32 transplant, which allows the immune system to fight the remaining HIV without simply repeating the infectious cycle again, changing the outcome of the immune cell : HIV interaction.
Question: Are there other ways for HIV to enter cells and infect them?
Answer: Due to its high mutation potential, HIV is not one static entity, and separate strains of the virus exist with different affinities to different entry receptors. Broadly speaking, an R5 strain of HIV would be more likely to enter cells which express both CD4 and CCR5, whereas an X4 strain of the virus would be more likely to enter a cell which expresses CD4 and CXCR4. While the London, Berlin, New York, City of Hope, and Dusseldorf patients show the potential for CCR5-negative immune cells as a functional cure for HIV, the Essen Patient provides an important counterexample, since this patient became populated by an X4 strain of the virus following a bone marrow transplant with CCR5Δ32/Δ32 cells. Accordingly, it stands to reason that attempts to cure HIV using immune cells which have been given additional layers of protection may be more effective than attempts which only deplete CCR5.
Question: How can we apply these lessons to make a widely available HIV cure?
Answer: The bone marrow transplant methods have successfully proved the concept of an HIV cure, but in order to roll these lessons into a widely available HIV cure, we need to address a few concerns:
- Bone marrow transplants are involved, invasive and have non-negligible risks.
- Donor cells with the CCR5Δ32/Δ32 genotype are rare, and the donor and the recipient need to match to a degree.
- The Essen patient shows us that we may want to deliver additional HIV countermeasures for more complete protection.
